Biology
Investigation:
The
Response of Blowfly Larvae to Light
Background Information
This section contains general information about blowflies relevant to this investigation, leading to the reasoning of a prediction in the next section.
 Blowflies
Blowflies
Blowflies are large-eyed flies, e.g. bluebottles Calliphora spp. and greenbottle Lucilia sericata. They lay their eggs in decaying meat and other foodstuffs, or in the case of greenbottle and L. cuprina (shown in Figure 5 on Page 13) in living sheep! The lifecycle of the fly that I will be using for my experiments (bluebottles) is shown in Figure 1. The numbers on the inside indicate the approximate days elapsing. Bluebottle is a name given to two similar species of true flies, Calliphora erythrocephala and C. vomitora. They have a four-stage life (metamorphosis) cycle, consisting of an egg stage, hatching into a larval stage, which metamorphoses into the pupa, from which the adult (imago) eventually hatches.
Each female bluebottle fly lays about 600 eggs, which hatch in about a day. Under favourable conditions the larvae pupate after in-between four days and a week, and emerge as adults a fortnight later, after twelve to seventy-two hours as pupae. This is summarised in Figure 1.
As the larva hatches out of the egg, it’s sole purpose is to feed so that it has enough energy for the metamorphosis during the non-feeding pupal stage. At the larval stage, a developing bluebottle has no protection from radiation, and it's white skin would make it stand out strongly to predators. Later towards the pupal stage it develops protective pigment, and changes to a less conspicuous brown colour.
As the eggs are laid in a foodstuff, it is only natural that it would be beneficial to the larva to stay there in the nutrient-rich, insulated, moist, and predator-free environment until it has enough energy and it pupates, and develops into a fly, when it requires to be in the open. It has, needless to say (nature is almost always perfect), evolved a mechanism to satisfy these changing needs.
Responses to
light
 Innate
behaviour, or instinct, is generally taken to be pattern of behaviour
elicited by specific stimuli and fulfilling vital needs of an organism. It is
demonstrated in its purest form by many lower animals, including insects.
Innate
behaviour, or instinct, is generally taken to be pattern of behaviour
elicited by specific stimuli and fulfilling vital needs of an organism. It is
demonstrated in its purest form by many lower animals, including insects.
The two types of behaviour in response to light which insects are capable of are:
· Phototaxis- movement of the whole organism towards light (positive phototactic response), or away from light (negative phototactic response)
·
Photokinesis-
the increase of rate of movement
and/or change in direction of
movement of the whole organism
Blowfly larvae have photoreceptors on each side of their heads, as shown in Figure 2. To gauge from which direction light comes, they move their heads from one side to the other. If they were to merely increase their rate of movement and/or change in direction of movement with light intensity (a kinesis), should they be on the surface, or reach the surface, they would instantly make themselves vulnerable to predators. This kind of movement would be too easy for predators to spot, although it stops when the organism is buried deep enough, to be of a good advantage to them. A change in allele frequencies resulting from their vulnerability in this situation would rather favour the evolution of direct movement away from the light (taxis). The ideal would be if the movement was away from the light source, and slowed down as the maggot reached the centre of its abode, where the light intensity was at its least, so that it stayed there. This is a negative phototactic response
Prediction
Based on the reasoning above, here is what I think will happen.
I therefore predict that the larvae will demonstrate a negative phototactic response. This will mean that they will:
· Move away from a light source
· Move away faster, the greater the intensity of the light
· Move away at a smaller angle to the direction of the light, the greater the intensity of the light
· Respond more strongly to more damaging (higher frequency and therefore energy) wavelengths, i.e. those closer to the Ultra-Violet end of the spectrum of visible light.
Plan
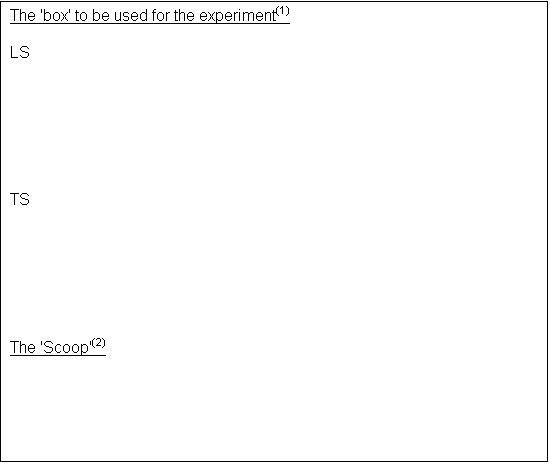 To investigate the validity of the above
theory, I have devised the following plan. (The numbers in superscript reference their justification
later in this document.)
To investigate the validity of the above
theory, I have devised the following plan. (The numbers in superscript reference their justification
later in this document.)
OUTLINE: I intend to take five blowfly larvae and subject them to high and low light intensities and different frequencies, and record the direction and magnitude of their response.
Apparatus:
· Black-lined box
§ Approximately 30 by 20 cm
§ With a shelve and mirrors at one narrow end, as shown
§ Lined with matt black paper and with a smooth black bottom(3) (with 1cm2 grid)
· Light source- neon strip lamp(4)
· Light Meter probe on a Logit box(5)
· Stop Clock/Timer(6)
· Narrow paper scoop
· Container to keep larvae in fridge(7)
· 40 blowfly larvae
Chronological order of the experiment:
· Set up apparatus as shown
· Black out room, so that there is just enough light to see by, but not enough to affect experiment.(8)
· Get five maggots out of the fridge, as needed for one experiment(9)
· Allow them to warm to room temperature(10)
· Set the variable resistor to the correct intensity, or fit filters, using light meter to check the value of the light intensity(11)
· Switch lights in room off
· Pick a larva up using the narrow paper scoop, with the head at the front end
· Place the larva in the centre point of the base of the box, its right side facing the light source, shutting the lid and starting the timer at the same time as switching the lamp on
· After 10, 20, and 30 seconds(12), note the position of the larva on the grid by looking through the peephole, recording the co-ordinates on the table shown below.
· Do this with all 5 larvae(13)
· Repeat this with red, green and blue filters, alternately, using the voltage pack to vary the output of the bulb, ensuring equal intensity readings on the light meter(14)
· Repeat this with unfiltered light of 2, 3, 4 & 5 times the light intensity(15)
This is the table on which I will record my results for each colour/intensity:
|
Larva |
Quality |
Temperature |
Co-ordinates 10 |
Co-ordinates 20 |
Co-ordinates 30 |
|
1 |
White |
|
|
|
|
|
2 |
White |
|
|
|
|
|
3 |
White |
|
|
|
|
|
4 |
White |
|
|
|
|
|
5 |
White |
|
|
|
|
|
1 |
Red |
|
|
|
|
|
2 |
Red |
|
|
|
|
|
3 |
Red |
|
|
|
|
|
4 |
Red |
|
|
|
|
|
5 |
Red |
|
|
|
|
|
1 |
Blue |
|
|
|
|
|
2 |
Blue |
|
|
|
|
|
3 |
Blue |
|
|
|
|
|
4 |
Blue |
|
|
|
|
|
5 |
Blue |
|
|
|
|
|
Larva |
Intensity |
Temperature |
Co-ordinates 10 |
Co-ordinates 20 |
Co-ordinates 30 |
|
1 |
1 |
|
|
|
|
|
2 |
1 |
|
|
|
|
|
3 |
1 |
|
|
|
|
|
4 |
1 |
|
|
|
|
|
5 |
1 |
|
|
|
|
|
1 |
2 |
|
|
|
|
|
2 |
2 |
|
|
|
|
|
3 |
2 |
|
|
|
|
|
4 |
2 |
|
|
|
|
|
5 |
2 |
|
|
|
|
|
1 |
3 |
|
|
|
|
|
2 |
3 |
|
|
|
|
|
3 |
3 |
|
|
|
|
|
4 |
3 |
|
|
|
|
|
5 |
3 |
|
|
|
|
Etc.
I will carry out the following statistical analysis on my results (on the spread sheet, together with the results):
· Use trigonometry to calculate the angle at which the larvae moved initially, intermediately, and finally(16), the direction being in the form of the number of degrees by which the path of the larva differed from the direction of light
Tan q
= X-component of movement
Y-component of movement
·
 Compare my results to the outcome predicted
by the null hypothesis using the chi-squared test to check their validity.(17)
Compare my results to the outcome predicted
by the null hypothesis using the chi-squared test to check their validity.(17)
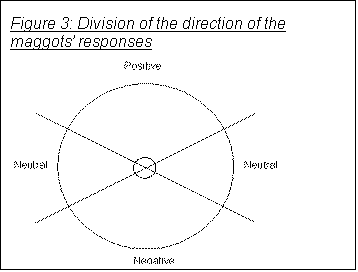
To do this I must first use the results of the above trigonometrical calculation to split the responses up into groups: those which showed positive phototaxis, those showing negative phototaxis, and those showing a neutral response, as shown in Figure 3..(17) The direction marked as positive is the one facing the light- the direction towards 40 on the 1cm2 grid. Positive and negative are 120°, the two neutral areas 60°.
The Chi-squared test
This can be used to analyse results for the degree of variation between a set of data and the expected outcome to show the validity of conclusions. I will use it to compare the results that I get to the results that I would expect if the movement of the larvae were purely random. The size of the number calculated will tell me how sure I can be that it is not purely random and therefore that it is reasonable to conclude an affect of the light on the larvae.
The equation is:
![]()
Where: x2= Chi-squared value
Σ= “sum of“
O= Occurrence of one species together with another
E= Expected value for the latter
As the angle is the same for each
the positive, negative and neutral areas (see Figure 3), the null hypothesis would predict that an equal number
of maggots would go in each of the directions positive, negative, and neutral. 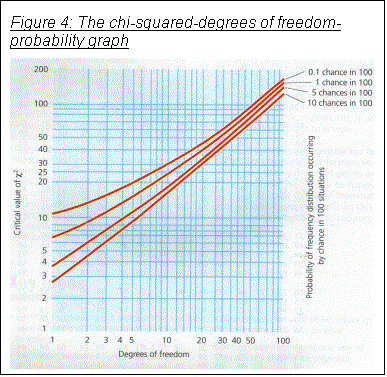 The degree of confidence can then be read off
a graph such as that shown in Figure 4.
This shows that if the probability is bigger than 3.84, I can be 95% sure that
there is a significant difference my readings and the results predicted by the
null hypothesis, and the null hypothesis can be rejected.
The degree of confidence can then be read off
a graph such as that shown in Figure 4.
This shows that if the probability is bigger than 3.84, I can be 95% sure that
there is a significant difference my readings and the results predicted by the
null hypothesis, and the null hypothesis can be rejected.
· Use standard deviation to check the validity of my results, as explained below.(18) This will also give me comparable values of the randomness of the data with a decrease in intensity, which may be a measure of how easily the maggots detect the light.
Standard Deviation
This is used to analyse the validity of the results. The greater the standard deviation of a set of data, the less valid the mean value is.
The equation for S.D. is:
![]()
Where: s= standard deviation
S= “the sum of“
n2 = the individual values of n, the angle or speed
N2= the mean of the values of n (the sum of them divided by the number of them), squared
x= number of values of n in the data
Therefore the standard deviation is the route of ((the sum of all values of n2 minus the mean of n2) divided by x, the number of values of n in the data).
I will plot the following graphs of my results:
· The variation of speed of movement (the velocity is worked out from the distance moved divided by the time taken) with light quality (velocity-wavelength)(19)
· The variation of direction of movement with light quality (degrees from normal-wavelength).(20)
· The effect of increasing the light intensity on the above factors (velocity-light intensity, degrees from normal-intensity).(21)
· The percentage of larvae showing a (a)positive, (b)negative and (c)neutral response.(22) at the different wavelengths and frequencies.
This last one will be in the form of a bar chart of percentages; the others will be scatter graphs.
Justifications
These are the reasons that I made the choices that I did. The numbers correspond to those in subscript in the plan.
1) The box is designed not to let any light from outside to flood in. This could cause the maggots to respond to the external light, rather than the intended source.
2) The scoop is to prevent injury to the maggot. If it is damaged, its responses may be lessened. Tweezers and other metal implements, fingers and spoons may easily cause injury. The scoop is designed not to.
3) Matt black is the most absorbent (least reflective) surface. I will line the walls of the box with it to increase the light intensity gradient. This also makes the light more one-directional. It must be flat-bottomed to prevent the maggots from moving in a particular direction purely because of a physical gradient.
4) Neon strip lamps emit much less heat than filament lamps, as they are more efficient. This reduces the possibility of maggots responding to I.R. stimulus.
5) The light meter is to ensure that when I double the intensity, the spot that the maggot starts on is actually receiving twice the intensity of light, otherwise the comparison of response to an increase is only quantitative.
6) Stop clocks are an accurate and easy-to-read way of timing the intervals between readings. They are simple to start and are free-standing.
7) The larvae need to be kept in the fridge during the two days over which I will do the experiments. This is because the lower temperature lowers the effectiveness of enzymes and therefore slows their metabolism down, so that they do not start pupating and hatching during the experiment.
8) Maggots’ response to extraneous light could affect the results. A way of minimising this is to work in a dimly lit room.
9) Only taking enough maggots out as will be used in the experiments further slows their development into pupae.
10) If they are used cold (just after they have come out of the fridge)
a) They will be warming up , so there will be a difference between the results of each individual maggot
b) They will not be as active, so their responses may be too slow to be recorded or the size of them may make them appear random.
11) Setting the variable resistor before the experiment starts prevents having to set the value in the dark, or switching it on to find it is on the wrong setting. Making sure that there is the same amount of light falling on the larvae with all the filters took into account the varying absorbency of the neon light (see point 14).
12) I have recorded the co-ordinates at different times to enable me to
a) plot graphs of the effect of wavelength and intensity on the rate of movement of larvae and;
b) look at the results afterwards and explain any anomalies by the overall behaviour of the maggots, from beginning to end.
13) The more re-runs the better, but more than five would take too long, and five is a good number because there is always a majority in the number going in one direction.
14) Checking that the light intensities for all three filters are the same is essential, as otherwise one cannot be sure whether they are responding to the change in intensity or wavelength.
15) I thought this would be a suitable range, as too low an intensity would get little response, too high an intensity may not be possible with the same lamp, and may affect the behaviour of the maggots, e.g. by blinding or dehydration them.
16) Having these measurements at different times gives a better picture of the general pattern of movement of the larva. Discussed more below.
17) The chi-squared test is a reliable test for statistical viability, and can be done on a spreadsheet. The results will be mathematically split up into these groups for easier graphical representation of the results. This will not be done during the experiment, as only having a record of the type of response of the larva gives little idea of where they have travelled with time, and therefore what the real response was. For example, if they took a long time to change direction but did eventually, this would not be shown if only their final destinations were recorded.
18) Standard deviation can easily be applied to any size of column or block of data, and the values can be compared easily.
19) This is to check my prediction that their response would be more rapid to more damaging wavelengths.
20) This is to check whether it made a bearing on their direction of movement: different direction is a different kind of response. Non-detectable wavelengths might result in random direction, as predicted by the null hypothesis.
21) This is to check my prediction that blowfly larvae would "move away faster, the greater the intensity of the light" and "move away at a smaller angle to the direction of the light, the greater the intensity of the light".
22) This is again to check my prediction that they would "respond more strongly to more damaging (higher frequency and therefore energy) wavelengths, i.e. those closer to the Ultra-Violet end of the spectrum of visible light". If this is true there will be less random or neutral movement at the higher energy end of the spectrum.
Limitations and Precautions
These are the factors that could render my investigation invalid, and the steps that I will take to minimise them
Heat pollution
Description: If there is a significant amount of I.R. radiation emitted from the source of light or any other stationary source, it is possible that the larvae respond to this rather than the visible light stimulus. This could have a large effect.
Steps taken to minimise it: Measure the temperature at the starting point for each of the different intensities to check whether it varies. Check every ten centimetres across the floor of the box to check if there is any difference. If so, record it next to the experiment starting at that point.
Variations in temperature
Description: The temperature in the lab varies from day to day, and throughout the day. This can not be avoided, but can be accounted for by taking the temperature every time.
Steps taken to minimise it: Take the temperature at the beginning of each experiment, and record it in the space in the table provided. This does not avoid the fact that, even after warming up for ten minutes they may still be at a different temperature to the box. This would, however, not really matter as all the maggots are in the box fr the same amount of time.
Light flooding
Description: If stray light of appreciable intensity falls on the larvae, they may respond to this rather than the one-directional strip lamp. This could cause the results to be invalid, as they would not be affected by the lamp whose intensiy is being measured.
Steps
taken to minimise it: I will avoid this problem by working in the dark and
with an enclosed box.
Pupating
Description: if the maggots get old enough, they may start to develop into the pre-pupal stage and their behaviour may begin to change.
Steps
taken to minimise it: Keep the maggots in the fridge and only out for as
long as necessary. Buy fresh maggots near the time of execution of the
experiment.
Paper does not simulate meat very well
Description: Meat is three-dimensional and maggots are three-dimensional. They have three dimensional receptors and move in a three-dimensional way. The movement that is being measured in this experiment is purely two-dimensional.
Steps
taken to minimise it: There is not much that can be done on this note,
apart from the darkness and one-directional light ensured by the black box.
Their movement on cardboard (in air) may be more difficult than that in a
firmer medium.
Small number of maggots causing fluctuations
Description:
The more samples are taken, the less the extent to which random results affect
the averages.
Steps
taken to minimise it: The effect of this can be kept to a minimal by
keeping the number of maggots the same. There is not enough time within the
scope of this experiment to repeat the experiment more than five times. Using a
different larva each time increases the chances of having random results but
decreases their affect as the other larva in the same experiment show it to be
anomalous.
Maggot injury
Description:
If a larva is handled carelessly or are used for many consecutive experiments,
it may become damaged and no longer act in a manner typical of other larvae.
Steps
taken to minimise it: using the scoop, using a different larva for each
experiment and not dropping them should minimise this.
Safety Risks
· As we will be working in the darkness, to prevent accidents bags must be kept under desks, and the floor spaces must be clear.
· Wash hands after handling blowfly
· Do not allow the colour filters/box to become overheated, as there is a fire hazard with the cardboard box.
· Good laboratory practise- making sure implements are put in the right places, wires of apparatus are insulated and away from water, and so on.
· Prevent maggot loss by having tightly enclosed box
Modifications
- As it was difficult to ensure that the larvae faced in the same direction until I started the stopwatch, I recorded the direction that they were facing on an extra column on the table.
- The room could not be as dark as I had hoped.
- I moved to a smaller room with less people to cut down the variations in light levels from many peoples' experiments.
- I found that the variable resistor had no effect on the intensity of the neon strip lamp, due to its internal electronics. I therefore moved the starting point for the maggots to vary the intensity and recorded the relative intensity on the light meter, as well as their starting point, in an extra column on the table.
- I had to use a camera as a light meter, as the one that I was planning to use (a ‘Logit box’) was insufficiently sensitive.
- It was therefore not able to measure 2 times the light intensity, three times the light intensity etc , but increased the light level according to the sensitivity of the camera’s meter.
- I decided to make the positive
responses red on the table, the neutral
responses blue for easier reading. Note: a negative response is taken to be away from the light and
therefore a negative response to light (negative phototaxis/photokinesis).
Henceforth when I refer to "negative" and "positive"
this is what I mean.
Results:
The results are shown on the next few pages. First comes the main results page.
[To download the results in Microsoft Excel 95 format, click here, email me
to request for other formats. This digital version does not include trend
lines, which must be included on the graphs to show correct interpretation]
Errors, limitations and Anomalies Affecting Results
Errors that could have been introduced by my method are:
·
Time inaccuracies
are unlikely due to the use of a stopwatch. There could have been some error
introduced by the slightly varying time lag between reading the time on the
clock and noting the co-ordinates of the larva.
·
Parallax error
could have been a factor, as taking readings from a small hole in one end of
the box meant that the angle at which I viewed the larva changed as it moved,
so the parallax error changed during each experiment, meaning that there may be
slight inaccuracies in the readings.
· Percentage inaccuracies were fairly high, as the measurements were only to the nearest centimetre and the distances between one and twenty centimetres.
All of these factors were counteracted by using five maggots and taking the averages of the measurements at 10, 20 and 30 seconds.
The limitations and errors that could have affected my results are:
Pupating
Although only about five to ten percent of the maggots used were actually seen to be changing colour, it is very possible that a larger percentage were tending towards the pre-pupal stage, and their responses were beginning to change. This would explain the fact that, in all samples, there are some showing positive phototaxis, whereas the majority showed a negative response.
Heat pollution
The table of my results from measuring the variation of temperature within the box is shown below.
|
Control: temperatures table |
||
|
Temperature |
Distance from light/cm |
|
|
17 |
5 |
|
|
16 |
10 |
|
|
17 |
15 |
|
|
17 |
20 |
|
|
17 |
25 |
|
|
16 |
30 |
|
|
16 |
35 |
|
|
17 |
40 |
|
This table shows little variation. Any variation could have been caused by random fluctuations in the circuitry of the Logit box or a borderline temperature of 16.5 and random fluctuations in air temperature within the box. There is no trend with the distance away from the light (although I left it on for 10 minutes before taking these measurements) and therefore it can be concluded that there was no influence on the maggots of this type.
Variations in temperature
The temperatures at the time of experimentation are recorded on the table of results. There is little fluctuation.
Light flooding
As this appeared to be a potential problem, I moved to a smaller room with less people, as stated in the modifications.
Paper does not simulate meat very well
This is definitely a point for consideration.
Although this experiment proves the responses of maggots to light in this
experiment, the differences between this experiment and the natural habitat of
the Blowfly must be considered before it can be concluded that this behaviour
is typical of all blowfly everywhere.
Small number of maggots causing fluctuations
Although the more samples there are the more sure one can be, it appears that five was a big enough sample to adequately investigate their responses.
Maggot injury
As a special scoop as described in the plan was used for this experiment, it is unlikely that this was a factor, although this could be an explanation for a few anomalies, as there could have been some injuries from their being in a large container with all the other larvae and could have been injured at this stage.
The main anomalies for consideration are:
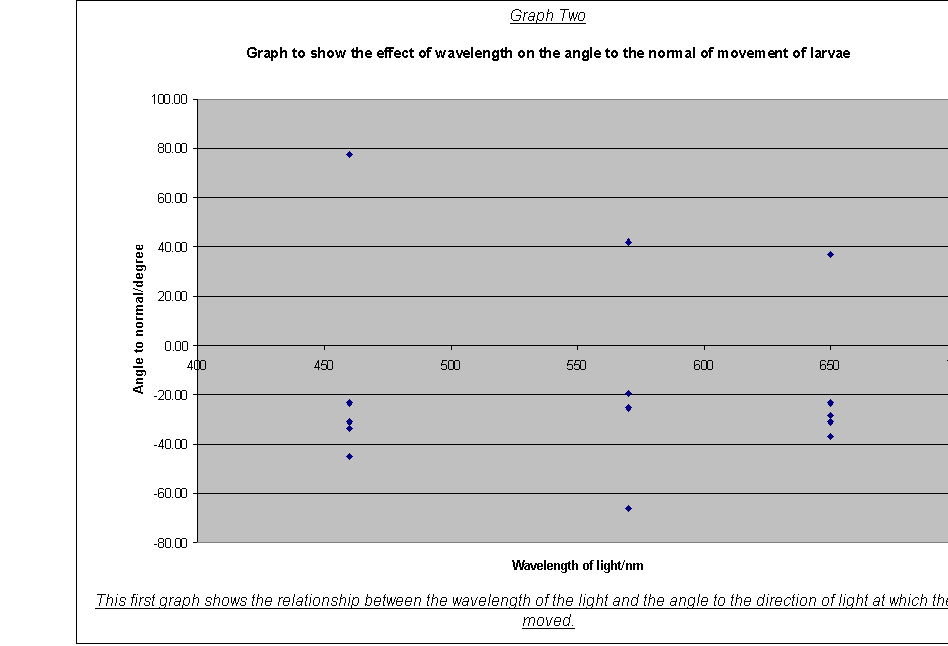
· Larva number 4, at wavelength 460nm: This showed a neutral response, moving at 77 degrees to the normal.
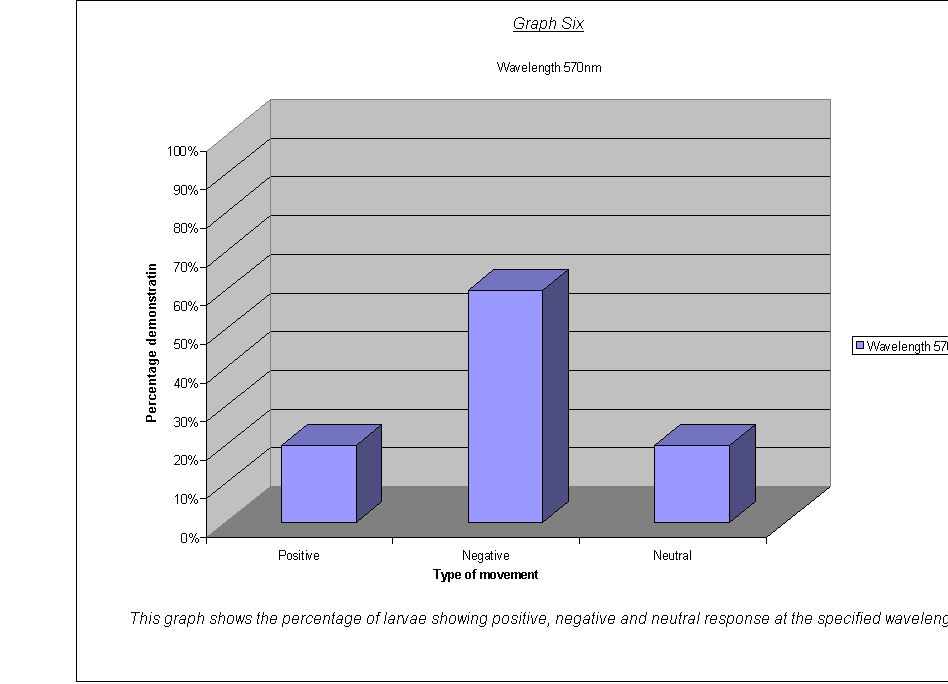
· Larva number 4, at wavelength 570nm: This showed a neutral response, moving at 66 degrees to the normal.
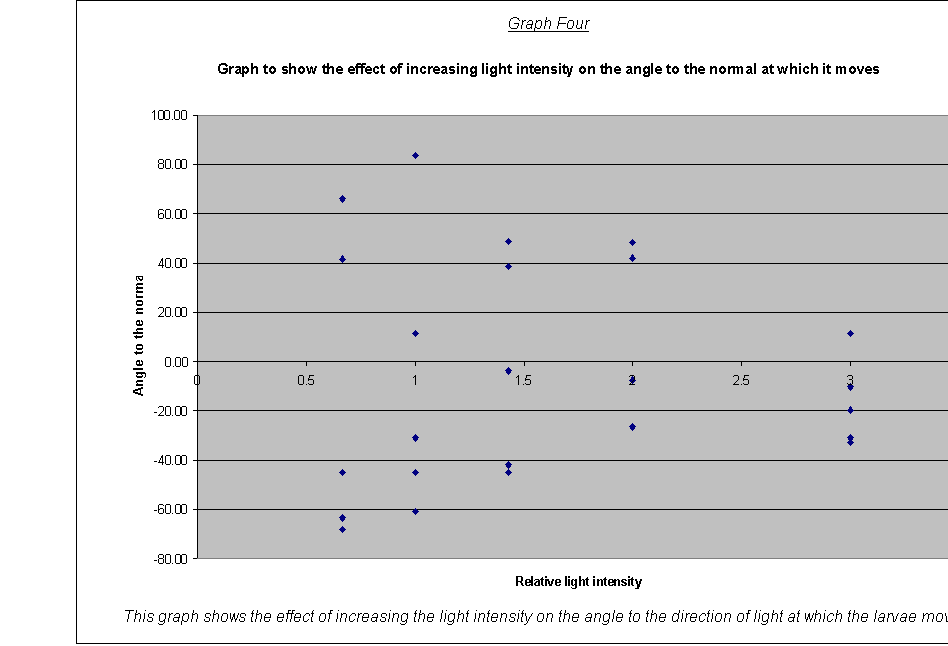
· Larva numbers 4&5, at relative intensity 1: These showed a neutral response, moving at 84 and 61 degrees to the normal.
· Larva numbers 2&3, at relative intensity 0.67: These showed a neutral response, moving at 68 and 63 degrees to the normal.
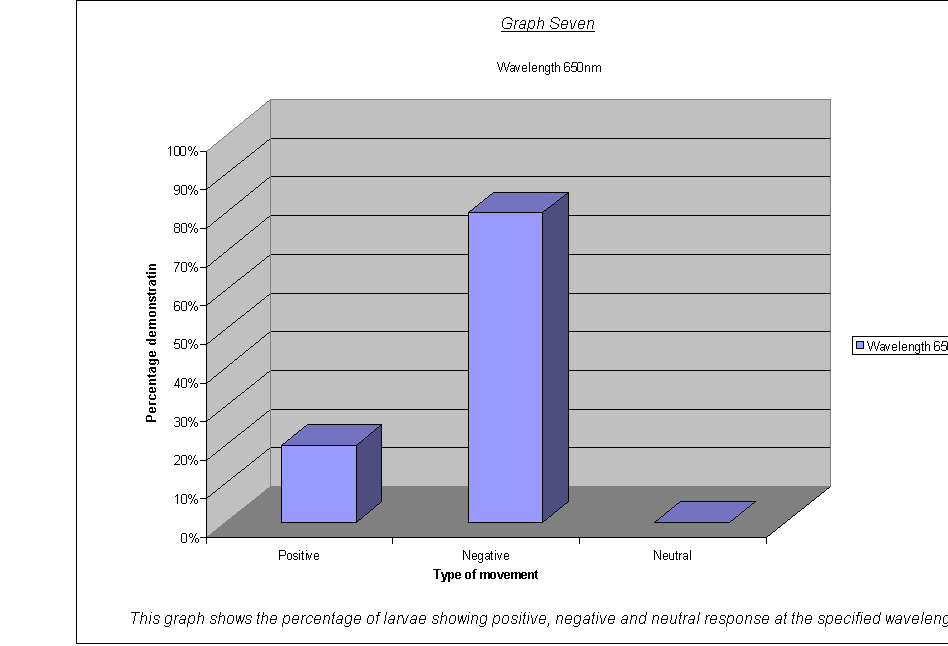
· I would have said Larva number 5, at wavelength 570nm, Larva number 1, at wavelength 650nm, Larva number 1, at relative intensity 3, Larva numbers 1&4, at relative intensity 2, Larva numbers 4&5, at relative intensity 1.43, Larva number 3, at relative intensity 1, and Larva numbers 1&5, at relative intensity 0.67, as they all showed a positive response, but the large number indicates a rule rather than an exception.
Conclusion
I found that the blowfly larvae demonstrated an innate negative phototactic response.
I found that an increase in the intensity of light did cause an increase in the phototactic response of blowfly larvae, but an increase in frequency of radiation did not produce such definite results.
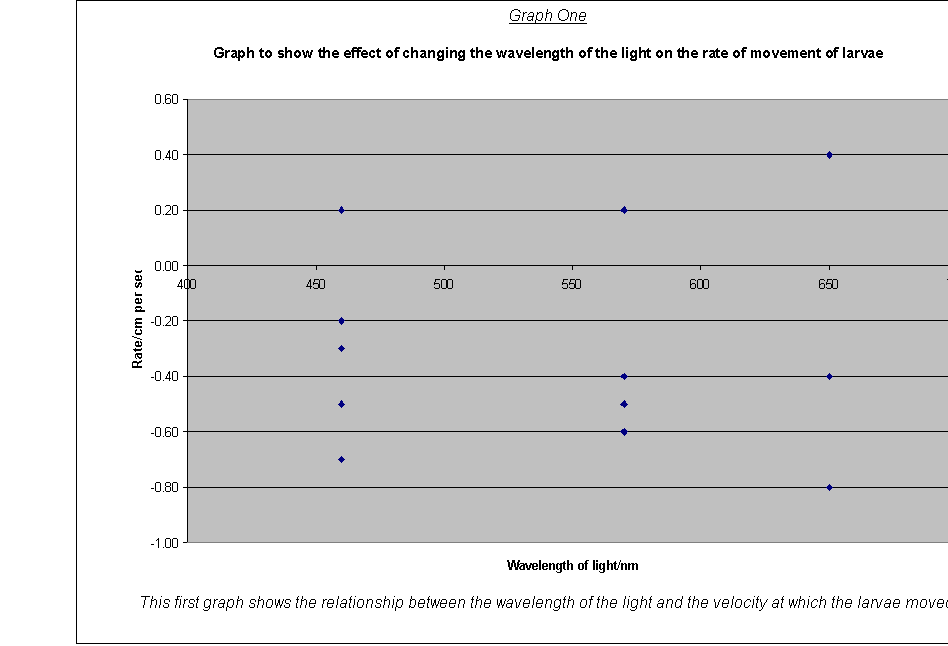
· Graph One: The graph of average rate of movement against wavelength showed that:
a) The larvae responded with most consistency to green light (570nm), although there was an anomaly with maggot five. This maggot moves very quickly sideways, but did not move much along the y-axis. It was possibly injured or otherwise just an anomaly.
b) The other wavelengths produced much more spread-out results- possibly a sign that the larvae are not sensitive to these frequencies.
· Graph Two: The graph of angle to normal against wavelength, however, showed more response to the other frequencies, but a extreme response for green. For example, the angles for blue ranged between –77.47 and 45 degrees, but for green they ranged between –41.99 (maggot five) and 66.04 degrees (maggot four), nearly staying completely within the ‘positive’ zone. This could show that the larvae are particularly sensitive to green light. The idea is backed up by my result for standard deviation- the deviation of movement for green light, at 0.31, is lower than that for the other frequencies.
The reason for this could be that, although higher frequency wavelengths are more damaging, they are not normally in the incident light falling on the larvae, as blue light is scattered more easily. Red light is barely detectable if the larvae are under vegetation, so green light is the important one. My results, however, do not differ so much between the three wavelengths that I tried, so the larvae may not be (so) sensitive to different wavelengths.
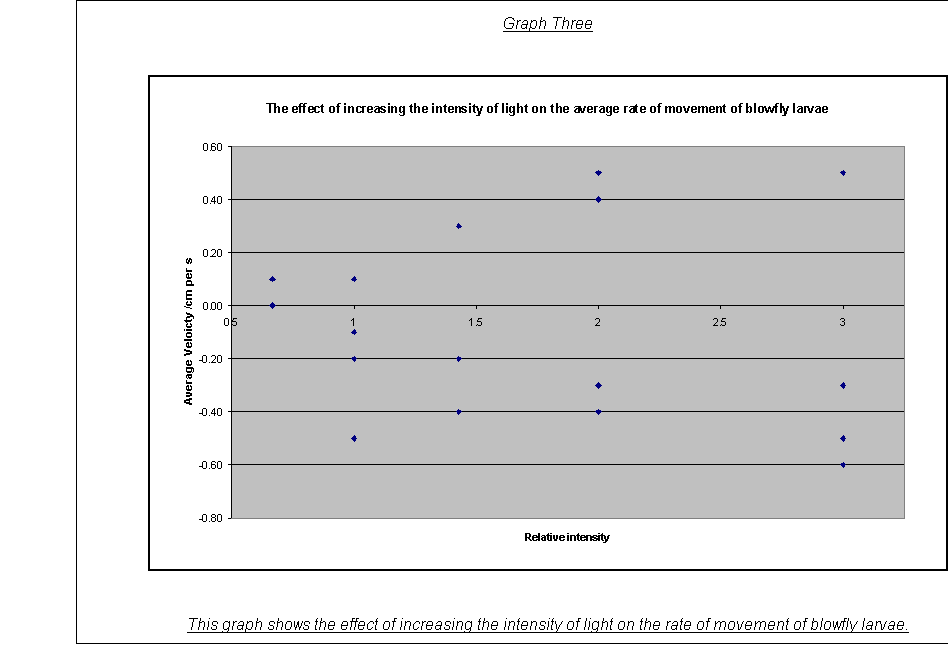
·
Graph Three:
The graph of the rate of movement against the relative light intensity showed
that the rate of movement is related by direct proportionality to the intensity
of the light i.e., that the larvae demonstrated a phototactic response. This
graphical observation is backed up by the standard deviation values, which are
smallest for the lowest intensities, as the distances moved are less, so the
extremes of movement are closer together. But the larvae were not always going
away from the light: for example maggot one for the relative intensity of 3.
This also demonstrates another point.
Whilst the larvae that moved towards the light at some intensities (e.g.
maggot 3 at relative intensity 1) appear to be wondering about slightly
randomly, maggot one at intensity 3 fits into a steady negative pattern: they
show both  negative
and a positive phototactic response (see below).
negative
and a positive phototactic response (see below).
· Graph Four The graph of the angle to the normal against relative light intensity showed this again: the more intense the light, the more focused the direction of the movement of larvae (the less got lost).
This can be explained by looking at the lifecycle of the blowfly in more detail. When the larvae need to develop into pupae (which are camouflaged brown and need to be on the surface so that the hatched flies (as shown in Figure 5) can fly away), directional movement will again be of great advantage, this time towards the light source. Photokinesis will again be of little value here, as in a large carcass it would take a long time to find the light, as random movement could result in going further down. So, although the larvae need to get in to the food to feed and protect itself whilst doing so, when it is ready to pupate, it must get back to the surface of its food to be able to hatch out of its pupal stage as a fly. The changeover in negative and positive phototaxis would enable this most efficiently as the larvae’s needs changed from needing to get away from the lighter surface, to needing to be on it. The phototaxis rather than photokinesis is the most efficient and inconspicuous way of doing this, as the goal is always either straight towards, or straight away from, the light.
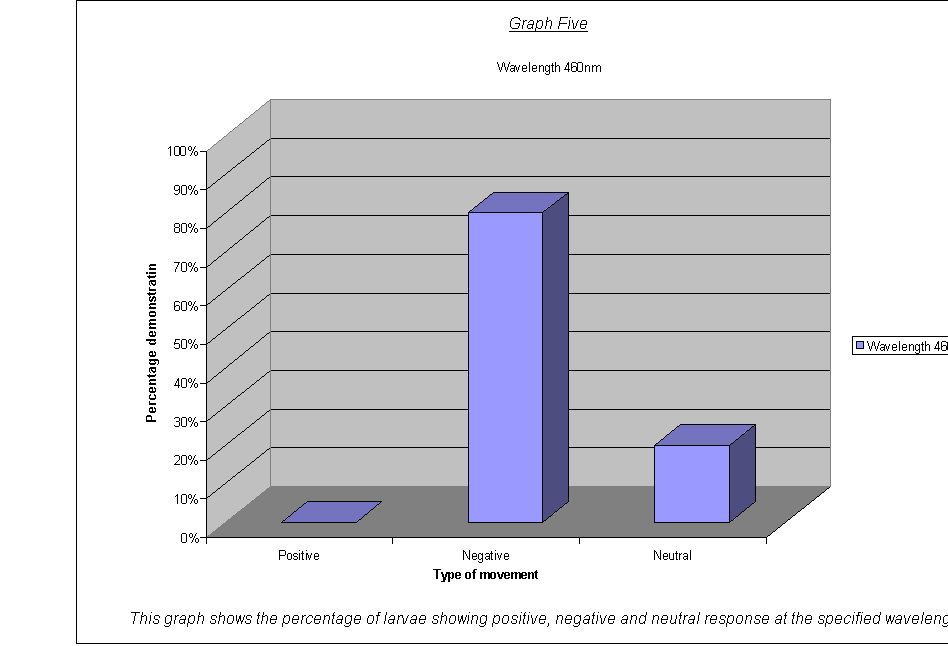
·
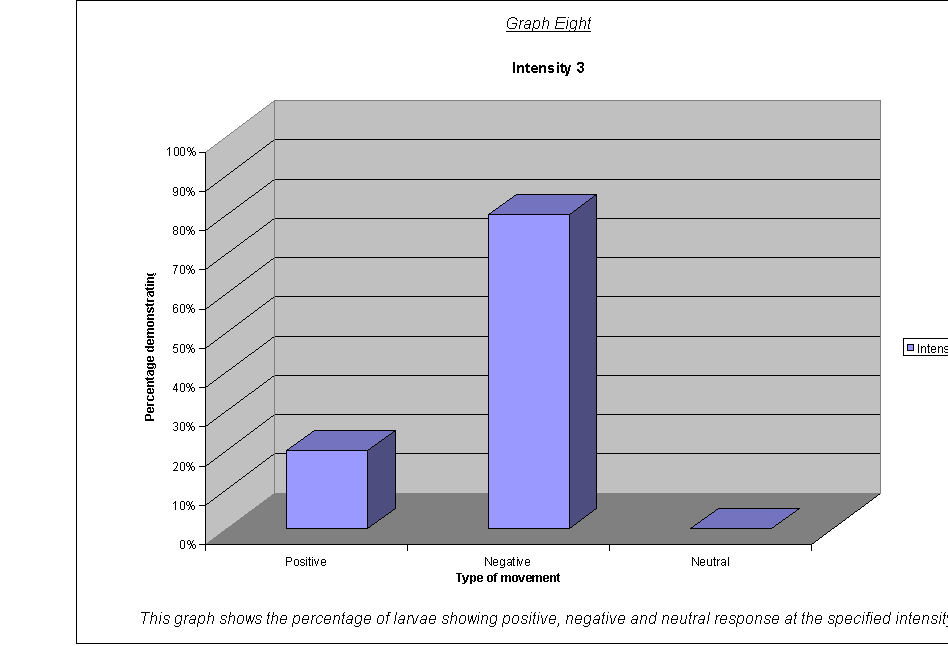
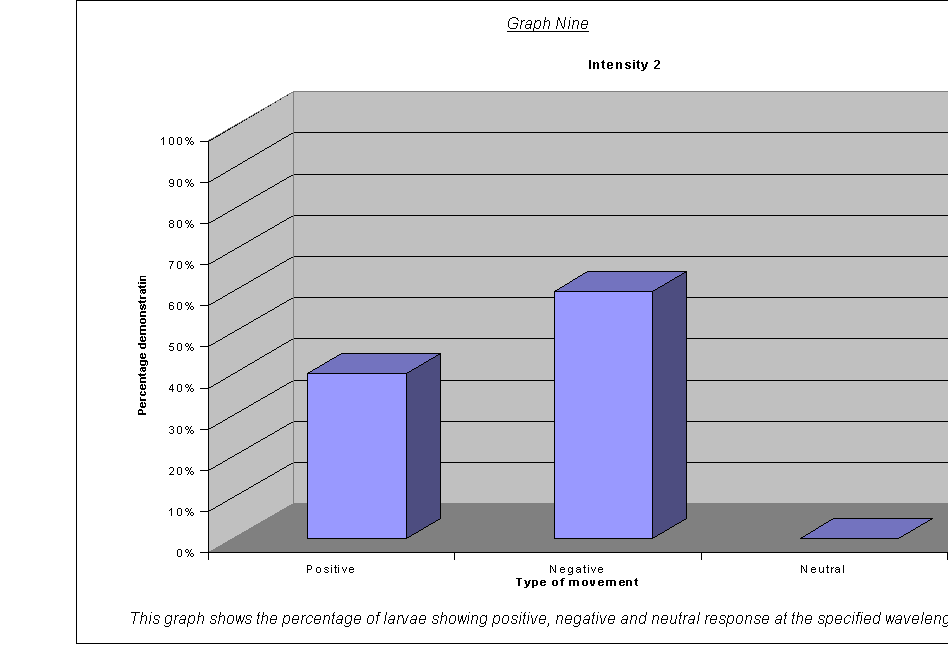
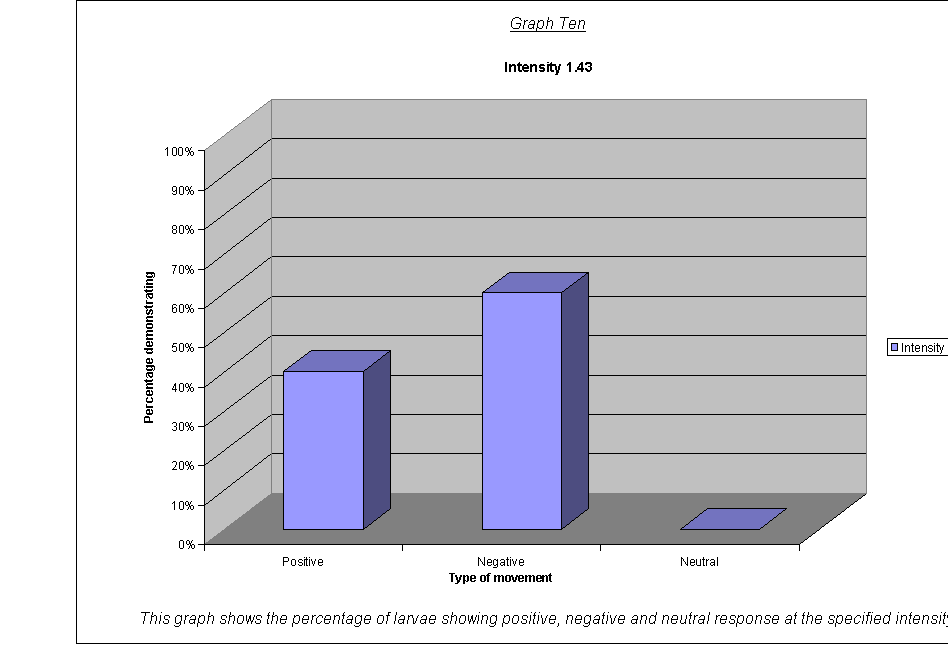
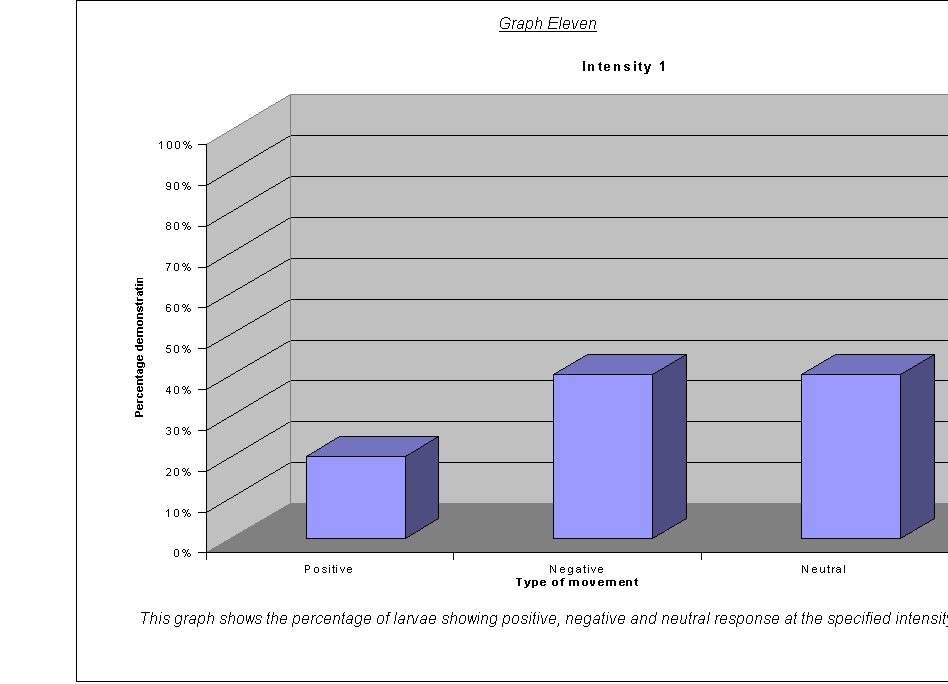
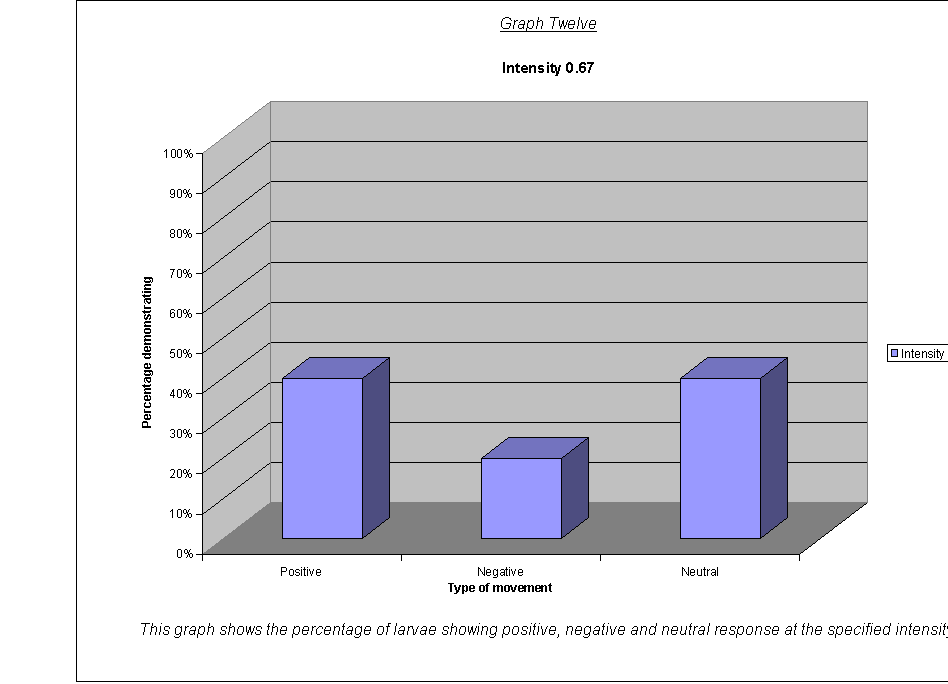
Graphs Eight
to Twelve: These show that for the highest intensities, the largest
proportion always demonstrated negative phototaxis. For intensity 1, the
negative was the same as the neutral response at 40%. For intensity 0.67, the
positive and neutral responses were larger than the negative, both including
40% of the larvae. This seems to suggest a threshold intensity: if the light is
not of a minimum intensity, the larvae may wander round, but not detect the
light sufficiently to be able to determine which direction to go in. They
therefore walk in a straight line (they start facing the side) and record as a
neutral response, or change direction for a slightly negative or positive
response. The reason that there appears to be a larger number of these
"wanderers" at low intensities could be either:
·
that there are more larvae at this intensity that can't
detect the light, therefore more demonstrate a random response
·
that the larvae at intensity 0.67 moved complete
randomly, and could not detect the light at all
The second suggestion is supported by the fact that the average rates of movement for the five larvae at intensity 0.67 were 0.1, 0.0, 0.0, 0.0, and 0.1. In other words, it seems very obvious that either the larvae could not actually detect the light at relative intensity 0.67, or their light sensors at this level were not sensitive enough to detect the difference between the level of light on their left and right sides.
It could be an advantage that their sensors are only triggered by certain levels of light, as, once they are far enough inside their piece of meat (or whatever), they need to stop and start feeding, so that they can start storing energy as soon as possible before they go into pupation. If they started running around at the first hint of light, they might spend all morning trying to get to the middle of the food, as when the sun rose all the larvae would fight for pole position! Evolution has therefore favoured a threshold frequency at an appropriate level, and the nervous response is only triggered when the stimulus is strong enough.
· Graphs Five to Seven: These all show negative phototaxis as the primary response. The nature of these type of graph combined with only having five repeats tends to exaggerate small results, for example having one little insignificant larva going a different direction means that it shows a block a fifth of the total height of the graph- not easily missed.
Conclusion Evaluation
Despite the anomalies discussed on page 10, I think that my conclusion is a valid one. The evidence for the existence of both negative and positive phototaxis, and the predominance of negative phototaxis is strong.
The chi-squared test, as shown below, demonstrates that the result of the variation between the results and those predicted by the null hypothesis was much greater than 3.84, so the null hypothesis can be rejected. Although the number was not so different for positive, there was a much smaller number of neutral responses than predicted, and a much larger number of negative responses.
|
Control:
Chi-squared test table |
|
|
|
|
|
||||
|
Type of
movement |
Observed
(O) |
|
Expected
(E) |
|
O - E |
((O - E )^2)/E |
|||
|
Positive |
|
10 |
|
13 |
|
11 |
8.53 |
||
|
Negative |
|
24 |
|
13 |
|
-3 |
0.83 |
||
|
Neutral |
|
6 |
|
13 |
|
-7 |
4.03 |
||
|
|
|
|
|
|
|
Total |
13 |
||
 The
evidence for the existence of a link between wavelength and sensitivity of the maggots
is, albeit weaker, still evident in all forms of analysis (angle to normal,
average rate of movement, and standard deviation), so I still think that there
is a link. This could, however, be investigated further, perhaps with a prism
to be able to vary the spectrum accurately. There were no known sources of
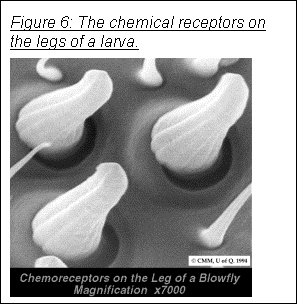
chemical pollution in the box. The only possibility is the Sellotape®. This
could have been detected by the chemoreceptors on the blowfly larvae’s legs (as
shown in Figure 6). This, however,
would have made any increase in light intensity have no effect on the larvae's
movement, and is therefore unlikely.
The
evidence for the existence of a link between wavelength and sensitivity of the maggots
is, albeit weaker, still evident in all forms of analysis (angle to normal,
average rate of movement, and standard deviation), so I still think that there
is a link. This could, however, be investigated further, perhaps with a prism
to be able to vary the spectrum accurately. There were no known sources of
chemical pollution in the box. The only possibility is the Sellotape®. This
could have been detected by the chemoreceptors on the blowfly larvae’s legs (as
shown in Figure 6). This, however,
would have made any increase in light intensity have no effect on the larvae's
movement, and is therefore unlikely.
As the
responses are innate (instinctive nervous responses rather than
conditioned/learned), they would not vary between Blowfly Larvae. I think that,
despite the differences between the natural and tested mediums, the conclusion
is valid, as a negative phototaxis would only be more strongly demonstrated in
an environment where it has evolved to move. Therefore it is safe to conclude
that this study is representative of all Blowfly Larvae.
Hits: